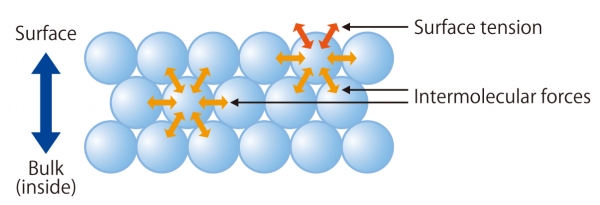
What is Surface Tension?
Intermolecular Force works between the molecules and the molecules of the liquid to try to minimize the surface area of the liquid. If Surface Tension is this force, the magnitude of the force that is exerted is a significant phenomenon such as Wettability.
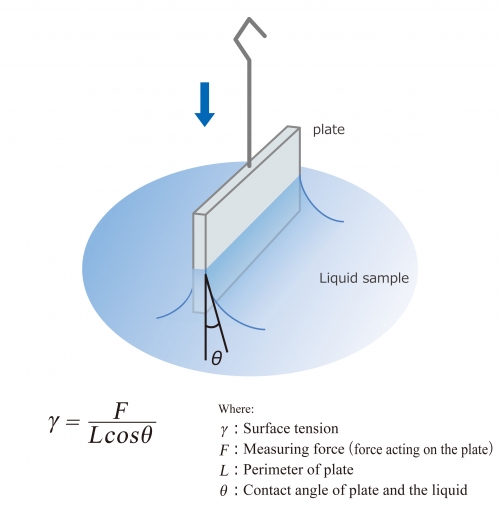
Wilhelmy Plate Method
When the measuring unit (Wilhelmy plate) makes contact with the surface of the liquid, the liquid will wet the Wilhelmy plate upwards. In this case, the surface tension acts along the perimeter of the plate and the liquid pulls in the plate. This method detects the pulling force and determines the surface tension.
Du Noüy Ring Method
The Noüy ring is made of platinum to ensure a contact angle of zero degrees with liquids. The method has a longer history than other modern measurement methods since its establishment and has been adopted by some industrial standards.
First, a ring parallel to the liquid surface is submerged. Then, the ring is gradually pulled vertically away from the surface. In this process, the surface tension of the liquid film attached to the ring generates a force on the ring. This force changes as the ring is drawn farther. Using the maximum value of this force, surface tension is determined by the following formula:

C in the formula is a correction factor and a constant corresponding to the ring size and liquid sample density. This factor corrects the effects of the tension direction and the liquid film's shape. Our DyneMaster series corrects them automatically with the aid of the Zuidema-Waters formula.
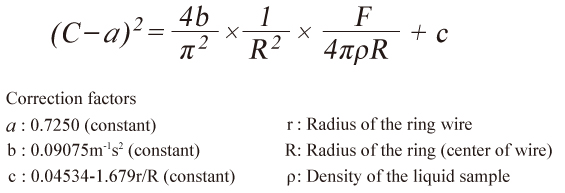
Lamella Length Measurement
One feature of surface tension measurements using the du Noüy tear-off method is that it also provides lamella length values. The lamella length indicates how long a liquid film can be prolonged before it detaches from the ring.
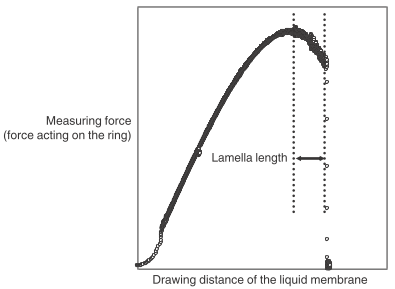
Drawing up the liquid film at high speed negatively influences its lamella length because it can cause the film to detach from the ring before reaching its full length. Therefore, a low constant speed and smooth movement of the sample stage are prerequisites for accurate measurements and good repeatability.
The lamella's length delivers information on the onset of curtain breakup of paint and coating films in curtain coating, foam stability, and antifoaming properties. It also indicates the pick-up ability of paints and coating liquids onto coating rolls.
Related Products
Pendant Drop Method
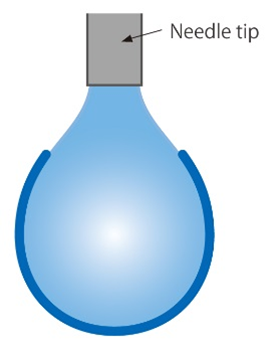
A liquid droplet suspended from a needle tip forms the characteristic shape of a pendant. It can be suspended in a gas phase or in another liquid. The shape of the pendant drop depends on the liquid volume (weight), the density difference Δρ between the phases, and the surface or interfacial tension.
The surface or interfacial tension can be determined by taking a photo image and analyzing the contour of the droplet’s shape.
The pendant drop method is suitable for measurements of highly viscous liquids, molten polymers, waxes, and solders.
Compared to other measurement methods, such as the Wilhelmy plate or du Noüy ring, the pendant drop method requires only small amounts of liquids.
Young-Laplace equation
The Young-Laplace equation uses the density difference between the phases, a calculated curve according to the Young-Laplace equation will be fitted onto the droplet’s contour.
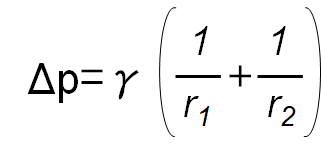
γ: Interfacial tension
Δρ: Density difference
r1, r2: Radii of curvature
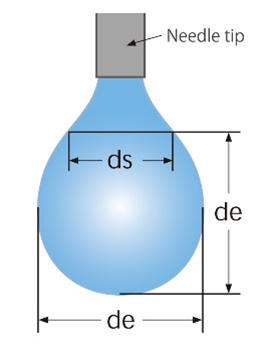
ds/de equation
The ds/de equation uses the interfacial tension, the equatorial diameter de, and the diameter ds, measured at the distance of de from the bottom of the droplet, to determine the surface tension.
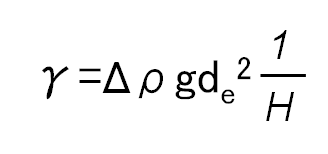
γ: Interfacial tension
Δρ: Density difference
g: Gravity
de: Equatorial diameter of the droplet
1/H: Correction factor derived from ds/de
Related Products
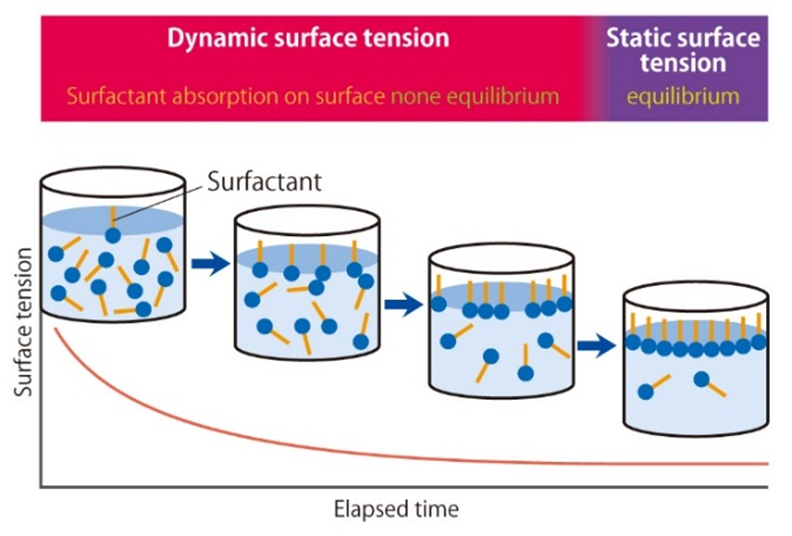
Dynamic Surface Tension
Surfactants lower surface or interfacial tension over time by adsorbing to the newly formed surface or interface. During the time from a freshly created surface to the equilibrium value of surface tension, dynamic surface tension can be measured; at the equilibrium state of the liquid, static surface tension can be measured. Detergents and coating solutions are used in processes where new surfaces or interfaces are constantly being created. Thus, determining the Dynamic Surface Tension is highly important.
Maximum Bubble Pressure Method
The maximum bubble pressure method calculates the surface tension by measuring the maximum pressure in an air bubble formed in a probe (glass capillary) immersed in a liquid.
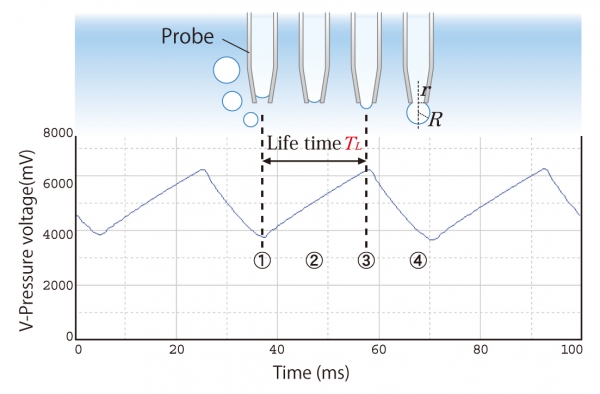
When pressurized air flows constantly through a capillary, the pressure inside the capillary changes in a regular cycle. →(1) to (4)
When the radius of the bubble curvature R equals the radius r of the capillary outlet, the pressure will reach its maximum. →(3)
The cycle from (1) to (3) is called lifetime or surface age.
Then, the bubble size increases quickly while the bubble pressure decreases until the bubble finally detaches from the capillary. →(4)
The cycle from (3) to (4) is called dead time.
A new bubble forms, and a new formation cycle begins from (1) to (4).
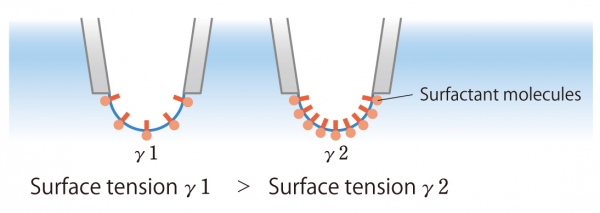
Using the Laplace equation, the bubble pressure method measures the maximum pressure and determines the surface tension. The number of surfactants that adsorb on the bubble surface during the lifetime reduces the surface tension.









