What is Surface Free Energy?
The contact angle θ of a liquid with a solid is the result of the interaction of the surface tension of the solid γS, the surface tension of the liquid γL, and the interfacial tension γSL between the liquid and the solid. The following Young's equation can express the relationship between liquids and solids:
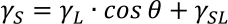
The following comparison describes the general relationship between surface tension and wettability:
Surface tension of liquid < Surface tension of solid ⇒ Small contact angle (good wettability)
Surface tension of liquid > Surface tension of solid ⇒ Large contact angle (insufficient wettability)
This relationship affects all applications in which controlling bonding and wetting properties is crucial.
However, cases where this relationship does not hold up are widespread. Here, using a method based on the concept that the surface free energy of solids is divided into different components effectively explains this discrepancy.
Surface Tension & Surface Free Energy
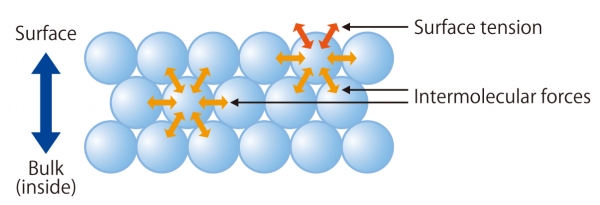
Surface tension results from the intermolecular force that acts between molecules. The figure on the right illustrates how these forces work differently on the molecules in bulk and at the liquid's surface.
The molecules in the bulk liquid are balanced as these molecules have cohesive forces acting equally in all directions. However, molecules on the surface only have intermolecular forces acting lateral and inward, pulling them inward. As a result, the liquid minimizes its surface area to the smallest possible, creating surface tension.
The effect of the excess energy on the surface, searching for a partner to attract to the atmosphere side, is why the wettability depends on the magnitude of surface tension.
Components of the total Surface Free Energy
The total surface tension of liquids and solids consists of two different components. The dispersive or disperse part on the one hand and the polar part on the other hand.
Whereas a surface tension can consist of only dispersion forces, only polar part surface tension does not exist. That is because dispersion forces act between all atoms and molecules.
Intermolecular forces comprise the Dispersive (London) force, the Orientation (Keesom) force, the Inductive (Debye) force, as well as Hydrogen bonds. The three forces except the hydrogen bonds are called van der Waals forces. Based on this assumption the total Surface Free Energy can be expressed with the following term:![]()
For phenomena where the relation between surface tension and wettability does not hold up, valuable information that can be drawn from the above-mentioned term with regard to the interactive forces between the different components of the total Surface Free Energy can help to solve challenging wetting behaviors.
| Dispersive (London) force | The dispersion forces are also called London forces and are part of the Van der Waals forces. London forces are the weakest intermolecular forces of all. Asymmetric distribution of electrons in the valence shell creates temporary (instantaneous) dipole in molecules and happens to all atoms or molecules. This event is also called polarization. |
| Orientation (Keesom) force | Dipole-dipole interactions are also called Keesom forces. These forces act between permanent dipoles forces which are created by differences in polarities between nearby molecules. These somewhat positive and somewhat negative charges cause molecules to attract each other. Dipole-dipole interactions tend to be weaker than hydrogen bonding forces. |
| Inductive (Debye) force | Dipole-induced dipole interactions are also called Debye forces. These forces are caused when a permanent polar molecule comes in close proximity to a nonpolar molecule and the static electricity of the polar molecule induces a temporary (instantaneous) dipole in the nonpolar molecule. The forces of attraction are very weak and can be neglected when considering surface tensions. |
| Hydrogen bonds | Hydrogen bonds are the strongest of the intermolecular forces and occur in molecules, such as F-H, O-H, and N-H. The high electronegativity of the elements F, O, and N in combination with H cause a strongly asymmetric distribution of the electron density. These created dipoles align with other dipole molecules and form interactions with a significantly bonding force. |
Example of the Surface Free Energy
The following is an example of the phenomenon in which the relationship between the magnitude of the surface tension value and the wettability does not hold. Despite the high surface tension of water (72.8mN/m), which is significantly higher than the surface tension of hexane (27.6mN/m), water spreads on the glass surface at the same level or even better than hexane. The reason for this is that the surface free energy components of water are close to the surface free energy components of glass, resulting in a low interfacial tension. This leads to good wettability and high adhesive force, despite the high surface tension of water. By introducing the concept of component division of surface free energy, wetting behavior and mechanism of wetting can be pursued in more depth, and the range of applications to control wettability can be expanded.
Methods to calculate the Surface Free Energy
The wettability between solid and liquid, and the adhesive force between solid and solid can be calculated by the following formulas:
Young equation: ![]()
Dupré equation:
From the above equations, the Young-Dupré equation can be obtained by substitution:
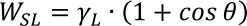
By measuring the surface tension of the liquid and the contact angle of that liquid with the solid, the work of adhesion between the liquid and solid can be calculated. The following example shows the calculation using the Kitazaki-Hata (Extended Fowkes) method:
Kitazaki-Hata (Extended Fowkes):
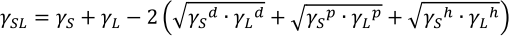
Dupré equation in Kitazaki-Hata:
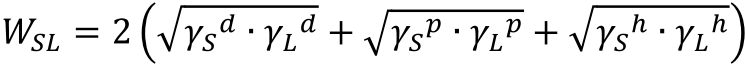

Young-Dupré equation in Kitazaki-Hata:
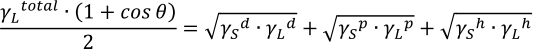
Where:

Using the Kitazaki-Hata (Extended Fowkes) theory, the surface free energy can be calculated from the contact angle results of three liquids with known dispersed, polar, and hydrogen bond components. The work of adhesion WSL of each component γd, γp, γh of both solid and liquid, and the interfacial free energy γSL between the solid and liquid can also be obtained. Methods that allow the calculation using only two liquids with known disperse and polar components also exist.
The following table shows the most common theories for surface free energy analysis:
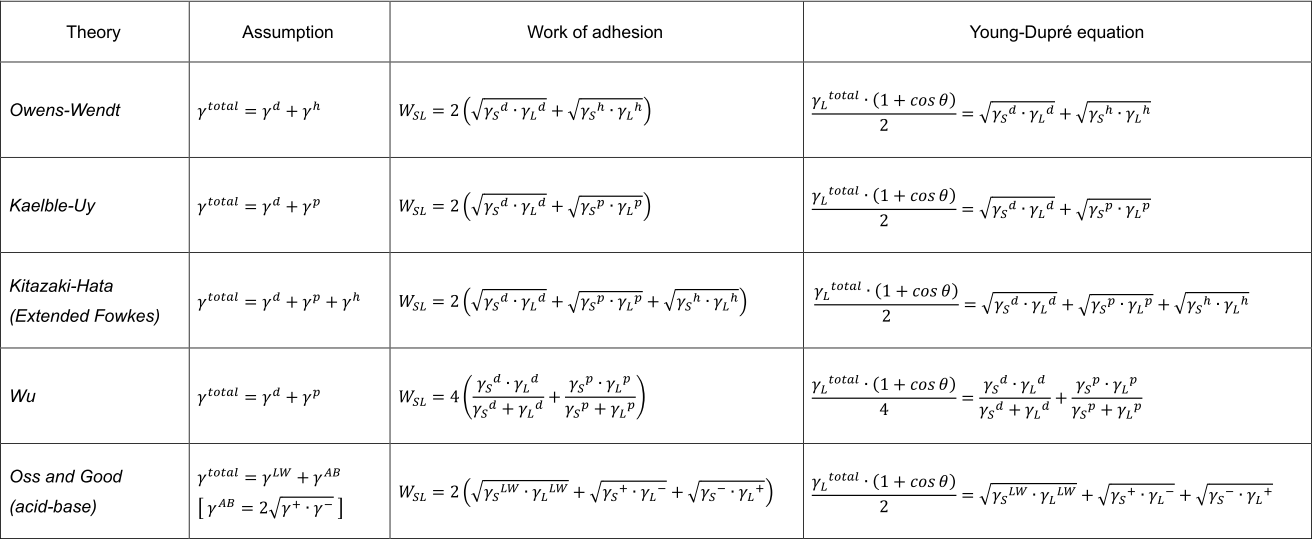
On the other hand, there are various theories about surface-free energy other than the above, and some ideas deny the component division. In that sense, it is hard to say that it is a well-established method as an analysis technology, and the fact is that the analyst himself has to search for the best method by trial and error. It is desirable not only to take the surface free energy analysis result to conclude but also to utilize it complementarily with other surface analysis methods such as XPS. In other words, it is realistic to use surface free energy analysis results as backup data to support specific predictions and conclusions.
Application Examples of Surface Free Energy
Reducing the Surface Free Energy of substrates by thin-film coating processes such as Physical Vapor Deposition (PVD) or Chemical Vapor Deposition (CVD)
- Improvement of mold release properties
- Improvement of friction and wear resistance of moving parts for car components
Increasing the Surface Free Energy of substrates by treatment with UV or Plasma, or by photocatalytic coatings
- Increase of adhesive properties of polymers
- Improvement of self-cleaning and anti-fouling abilities of outside walls, roofs, tiles in tunnels, etc.
- Improvement of anti-fogging characteristics by enhanced surface hydrophilicity







